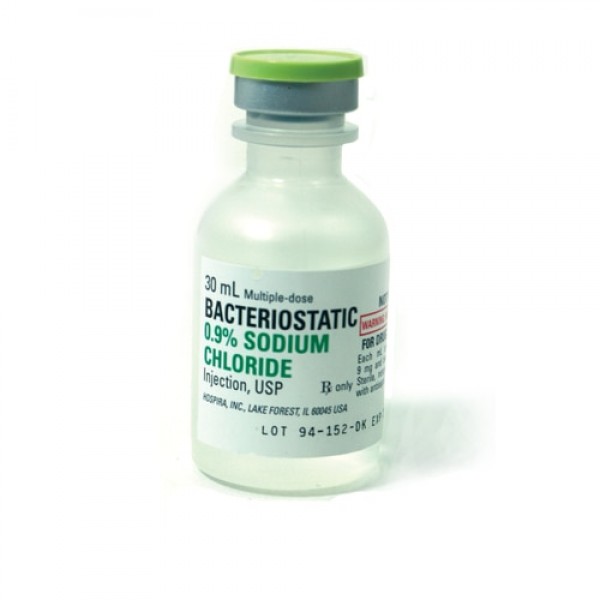
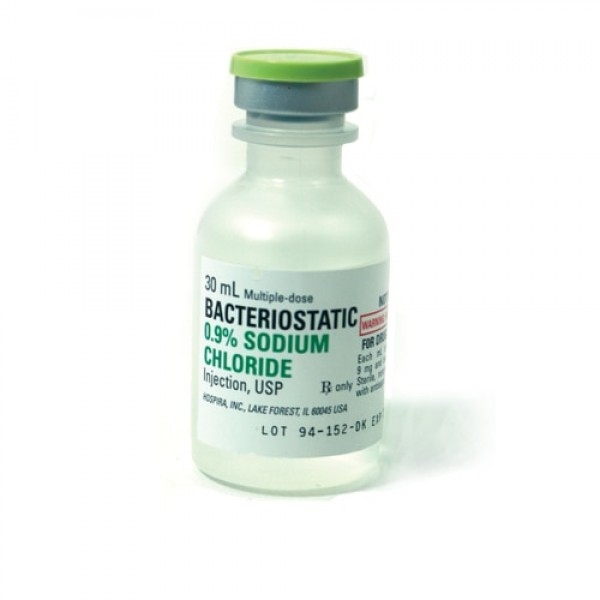
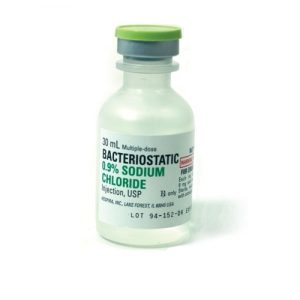
Bacteriostatic Sodium Chloride For Injection 20ml.
$12.00 Le prix initial était : $12.00.$5.00Le prix actuel est : $5.00.
-
United States dollar ($) - USD
-
Livre sterling (£) - GBP
-
Euro (€) - EUR
Bacteriostatic Sodium Chloride (normal saline) for Injection 20ml by Pfizer Injectables (formally Hospira Worldwide). Bacteriostatic Sodium Chloride is sterile, multiple-dose sodium chloride in water for injection. Each milliliter contains sodium chloride 9mg and 0.9% benzyl alcohol added as a bacteriostatic preservative that allows you to reuse repeated withdrawals for up to 28 days.
Description
Bacteriostatic Sodium Chloride for Injection 0.9% is a sterile, nonpyrogenic, isotonic solution of sodium chloride (saline solution) for injection. Each milliliter (mL) contains sodium chloride 9 mg and 0.9% (9 mg/mL) benzyl alcohol added as a bacteriostatic preservative. May contain hydrochloric acid for pH adjustment. It is supplied in a multiple-dose container from which repeated withdrawals may be made to dilute or dissolve drugs for medication. The pH is 5.0 (4.5 to 7.0).
Bacteriostatic Sodium Chloride for Injection is chemically designated NaCl, a white crystalline powder freely soluble in water.
The semi-rigid vial is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
Contraindications
Due to the potential toxicity of benzyl alcohol in neonates, solutions containing benzyl alcohol must not be used in this patient population.
Parenteral preparations with benzyl alcohol should not be used for fluid or sodium chloride replacement.
Parenteral preparations containing benzyl alcohol should not be used in epidural or spinal anesthetic procedures.
Bacteriostatic Sodium Chloride for Injection Warnings
Benzyl alcohol, a preservative in Bacteriostatic Sodium Chloride Injection, USP has been associated with toxicity in neonates. Data are unavailable on the toxicity of other preservatives in this age group. Preservative-free Sodium Chloride Injection should be used for flushing intravascular catheters. Where a sodium chloride solution is required for preparing or diluting medications for use in neonates, only preservative-free Sodium Chloride Injection should be used.
Bacteriostatic Sodium Chloride for Injection Precautions
Consult the manufacturer’s instructions for choice of vehicle, appropriate dilution or volume for dissolving the drugs to be injected, including the route and rate of injection.
Inspect reconstituted (diluted or dissolved) drugs for clarity (if soluble) and freedom from unexpected precipitation or discoloration prior to administration.
Pregnancy Category C. Animal reproduction studies have not been conducted with Bacteriostatic 0.9% Sodium Chloride Injection, USP. It is also not known whether Bacteriostatic 0.9% Sodium Chloride Injection containing additives can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Bacteriostatic 0.9% Sodium Chloride Injection containing additives should be given to a pregnant woman only if clearly needed.
Pediatric Use
The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. However, due to potential toxicity of benzyl alcohol in neonates, solutions containing benzyl alcohol are contraindicated in this patient population.
Drug Interactions
Some drugs for injection may be incompatible in a given vehicle, or when combined in the same vehicle or in a vehicle containing benzyl alcohol. Consult with pharmacist, if available.
Use aseptic technique for single or multiple entry and withdrawal from all containers.
When diluting or dissolving drugs, mix thoroughly and use promptly.
Do not store reconstituted solutions of drugs for injection unless otherwise directed by the manufacturer of the solute.
Do not use unless the solution is clear and seal intact.
Adverse Reactions
Reactions which may occur because of this solution, added drugs or the technique of reconstitution or administration include febrile response, local tenderness, abscess, tissue necrosis or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasation.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate countermeasures, and if possible, retrieve and save the remainder of the unused vehicle for examination.
Although adverse reactions to intravenous, intramuscular or subcutaneous injection of 0.9% benzyl alcohol are not known to occur in man, experimental studies of small volume parenteral preparations containing 0.9% benzyl alcohol in several species of animals have indicated that an estimated intravenous dose up to 30 mL may be safely given to an adult without toxic effects. Administration of an estimated 9 mL to a 6 kg neonate or infant is potentially capable of producing blood pressure changes.
Overdosage
Use only as a diluent or solvent. This parenteral preparation is unlikely to pose a threat of sodium chloride or fluid overload except possibly in neonates and very small infants. In the event these should occur, re-evaluate the patient and institute appropriate corrective measures.
Bacteriostatic Sodium Chloride Dosage and Administration
The volume of the preparation to be used for diluting or dissolving any drug for injection, is dependent on the vehicle concentration, dose and route of administration as recommended by the manufacturer.
Store:
20° 20 25°C (68 to 77°F)
Active ingredient:
benzyl alcohol C7H8O (type: aiha weel)
Molecular Formula:
C7H8O
Color:
clear, colorless
Container type:
Multiple Dose Plastic Fliptop Vial
Produits similaires
BUY BOTOX ONLINE
BUY BOTOX ONLINE
BUY BOTOX ONLINE
BUY BOTULINUM TOXINS
BUY BOTOX ONLINE
BUY BOTULINUM TOXINS
BUY BOTOX ONLINE
BUY BOTOX ONLINE